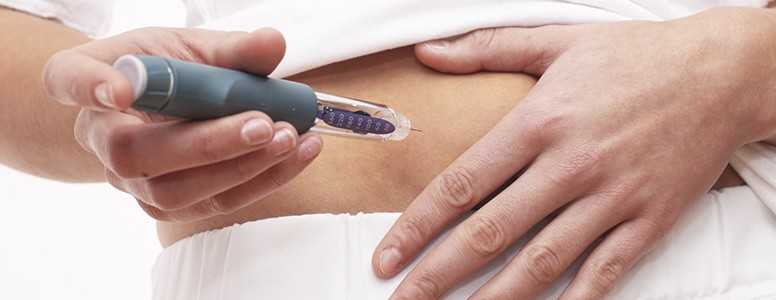
A new diabetes drug from Eli Lilly and Company, called Basaglar, has been approved by the U.S. Food and Drug Administration (FDA).
Basaglar is a long-acting insulin glargine that can improve glycemic control in adults with type 1 diabetes and type 2 diabetes, and pediatric patients with type 1. The drug received tentative FDA approval in August 2014, but has now been granted final approval.
According to Eli Lilly spokesman Greg Kueterma, Basaglar is a cheaper version of Sanofi’s top-selling drug Lantus. In September, Eli Lilly resolved a patent litigation with Sanofi to launch Basaglar in the United States in December 2016.
Basaglar was approved by the FDA after two clinical trials – which respectively enrolled 534 and 744 patients with type 1 and type 2 diabetes – demonstrated similar safety and effectiveness to Lantus, alongside certain Basaglar-specific data.
In 2014, Basaglar was approved in Europe as a “biosimilar” under the name Abrasia. The term “biosimilar” refers to the product being created from the same protein sequence as Lantus. The FDA has not approved Basaglar as a biosimilar product, instead it has been called a “follow-on” biologic.
How is Basaglar taken?
Basaglar is administered via injection subcutaneously once daily at any time of the day, but at the same time every day. Dosing should be individualised based on the patient’s requirements, but should not be used during episodes of hypoglycemia.
Basaglar can cause hypoglycemia, and all patients taking Basaglar should monitor their blood glucose levels. Additionally, insulin regimens should only be modified under medical supervision.
The most common side effects of Basaglar observed in clinical trials included hypoglycemia, injection site reactions, weight gain and allergic reaction.
Jean-Marc Guettier, M.D., director of the Division of Metabolism and Endocrinology Products in the FDA’s Centre for Drug Evaluation and Research, said: “Long-acting insulin products like insulin glargine play an important role in the treatment of types 1 and 2 diabetes mellitus, and [Wednesday’s] approval is expected to expand the availability of treatment options for health care professionals and patients.””
Is Basaglar available in the UK?
While Basaglar was approved as Abrasia in Europen, it was released in the UK in September 2015 as Abasaglar. Abasaglar is developed by Eli Lilly and Boehringer Ingelheim.
Abasaglar will reportedly be cheaper for the NHS than Lantus or Toujeo, a long-acting basal insulin from Sanofi.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.