Just a by the by, I have had 2 out of 3 sensors fail in the last 3 weeks. Tonight when I talked to the people at Medtronics about the latest failure, I asked them about the next generation sensor that is to replace this IMO woefully inadequate sensor we currently have (G4) and the very kind women I was talking to, suggested that the replacement for the G4 was still in the developmental and evaluation phase. MMMMMMM. That's my tiny little brain ticking over in frustrated anger.
-
Guest - w'd love to know what you think about the forum! Take the 2025 Survey »
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Medtronic guardian 4
- Thread starter SandieD
- Start Date
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Agree with what you are saying MoonCloudy. IMO, from a users perspective, the guardian 4 is so far behind the field, as to be not even in the race. As for still requiring additional tape to hold it in place and to now require a second person to help with the insertion and to only suggest the the back of the arm as a suitable and approved insertion site, well words almost fail me, almost. MoonCloudy, whoever at Medtronic thinks this a a generational improvement has no idea either about the current sensor market, nor what the actual end user requires from said sensors.
Regarding the Instinct sensor my friend, I am happy to be corrected, but I am unable to find any information that actually shows that it has been submitted for approval either in Asia, Europe, the States, or here in Australia. If anyone has any more information, I would appreciate it.
Stay well
Just a by the by, I have had 2 out of 3 sensors fail in the last 3 weeks. Tonight when I talked to the people at Medtronics about the latest failure, I asked them about the next generation sensor that is to replace this IMO woefully inadequate sensor we currently have (G4) and the very kind women I was talking to, suggested that the replacement for the G4 was still in the developmental and evaluation phase. MMMMMMM. That's my tiny little brain ticking over in frustrated anger.
Basically, I searched all kinds of information about CGM at Youtube channels where you can get a lots of info about the latest CGM details and updates from many Youtubers who are TD1 as well but none of them are using Medtronic and I am not surprised.
I watched and follow them every week and there is where I heard about the Simplera and Instinct.
To my understanding Simplera has been approved and Medtronic released the Simplera about 2 months ago?
Instinct will need some more time
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Basically, I searched all kinds of information about CGM at Youtube channels where you can get a lots of info about the latest CGM details and updates from many Youtubers who are TD1 as well but none of them are using Medtronic and I am not surprised.
I watched and follow them every week and there is where I heard about the Simplera and Instinct.
To my understanding Simplera has been approved and Medtronic released the Simplera about 2 months ago?
Instinct will need some more time
I don't really understand why a company can design a new sensor and a smart insulin pen, write software so that they can "talk" to each other and have that information available on an appropriate phone, yet at the same time seems to be so blind as to see that that sensor is exactly what is need to replace this really wonky G4 sensor. Do they really not get it, or are they so arrogant as to expect users of their pumps just to sit and suck it up while they open and attempt to dominate a new market segment, before looking after the clients they already have.
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
There you have it.....see, I am not the only one who is doubting about Medtronic and their way of doing at this moment when the competition among all kinds of sensors are already put on the market
Medtronic really needs to face their current situation and speed up with their progress when it comes to sensors.
Not to wait for another year or more for the Instinct before it ends up in our hands. The sooner the better
But MOST of all.....unpractical pride should be put aside and consider about their current users before they leave them as well sooner or later....Unfortunately, Medtronic is the only pump in my region, so I am stuck with it.
Though, 780G pump, itself is no problem, just those GS which makes everything so complicated
Medtronic...please please please....
Medtronic really needs to face their current situation and speed up with their progress when it comes to sensors.
Not to wait for another year or more for the Instinct before it ends up in our hands. The sooner the better
But MOST of all.....unpractical pride should be put aside and consider about their current users before they leave them as well sooner or later....Unfortunately, Medtronic is the only pump in my region, so I am stuck with it.
Though, 780G pump, itself is no problem, just those GS which makes everything so complicated
Medtronic...please please please....
If wishes were fishes we would all cast nets. I would think that a year would be a very optimistic expectation. perhaps they will take another look at the competition and see the sense of urgency that is developing and IMO, how far behind the pack they are being left. We shall see.
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Agree.If wishes were fishes we would all cast nets. I would think that a year would be a very optimistic expectation. perhaps they will take another look at the competition and see the sense of urgency that is developing and IMO, how far behind the pack they are being left. We shall see.
Time will tell.
Nevertheless....keep hoping for the best
I've been moved on to Medtronic 780 since November, and I've found the whole experience with the peripherals to be an absolute nightmare.
I think I've had two Guardian 4 sensors last the full 7 days. I've also had to replace the transmitter after a fortnight. The Guardian 4 is simply not fit for purpose. I can't believe it had any sort of user testing whatsoever. Having to mess around with the tapes to keep it in place drives me up the wall. I was using Freestyle Libre 3 before switching - they could stay happily attached for a fortnight.
I'm fed up of ringing Medtronic every week to report another failed sensor. One night I had to go through three before I got a functioning one. I asked my diabetes team to change my pump but they said I'm stuck with this one now until April 2025.
I think I've had two Guardian 4 sensors last the full 7 days. I've also had to replace the transmitter after a fortnight. The Guardian 4 is simply not fit for purpose. I can't believe it had any sort of user testing whatsoever. Having to mess around with the tapes to keep it in place drives me up the wall. I was using Freestyle Libre 3 before switching - they could stay happily attached for a fortnight.
I'm fed up of ringing Medtronic every week to report another failed sensor. One night I had to go through three before I got a functioning one. I asked my diabetes team to change my pump but they said I'm stuck with this one now until April 2025.
HairySmurf
Well-Known Member
- Messages
- 174
- Type of diabetes
- Type 2
- Treatment type
- Tablets (oral)
Disclaimer - I know nothing about this technology so I'm not certain this is helpful.I don't really understand why a company can design a new sensor and a smart insulin pen, write software so that they can "talk" to each other and have that information available on an appropriate phone, yet at the same time seems to be so blind as to see that that sensor is exactly what is need to replace this really wonky G4 sensor. Do they really not get it, or are they so arrogant as to expect users of their pumps just to sit and suck it up while they open and attempt to dominate a new market segment, before looking after the clients they already have.
After reading this thread and as I happen to know someone who works at Medtronic HQ I made contact asking if there were any plans to revise the Guardian 4. He does not work in the diabetes device division. I was provided with a little info and directed to this press release:
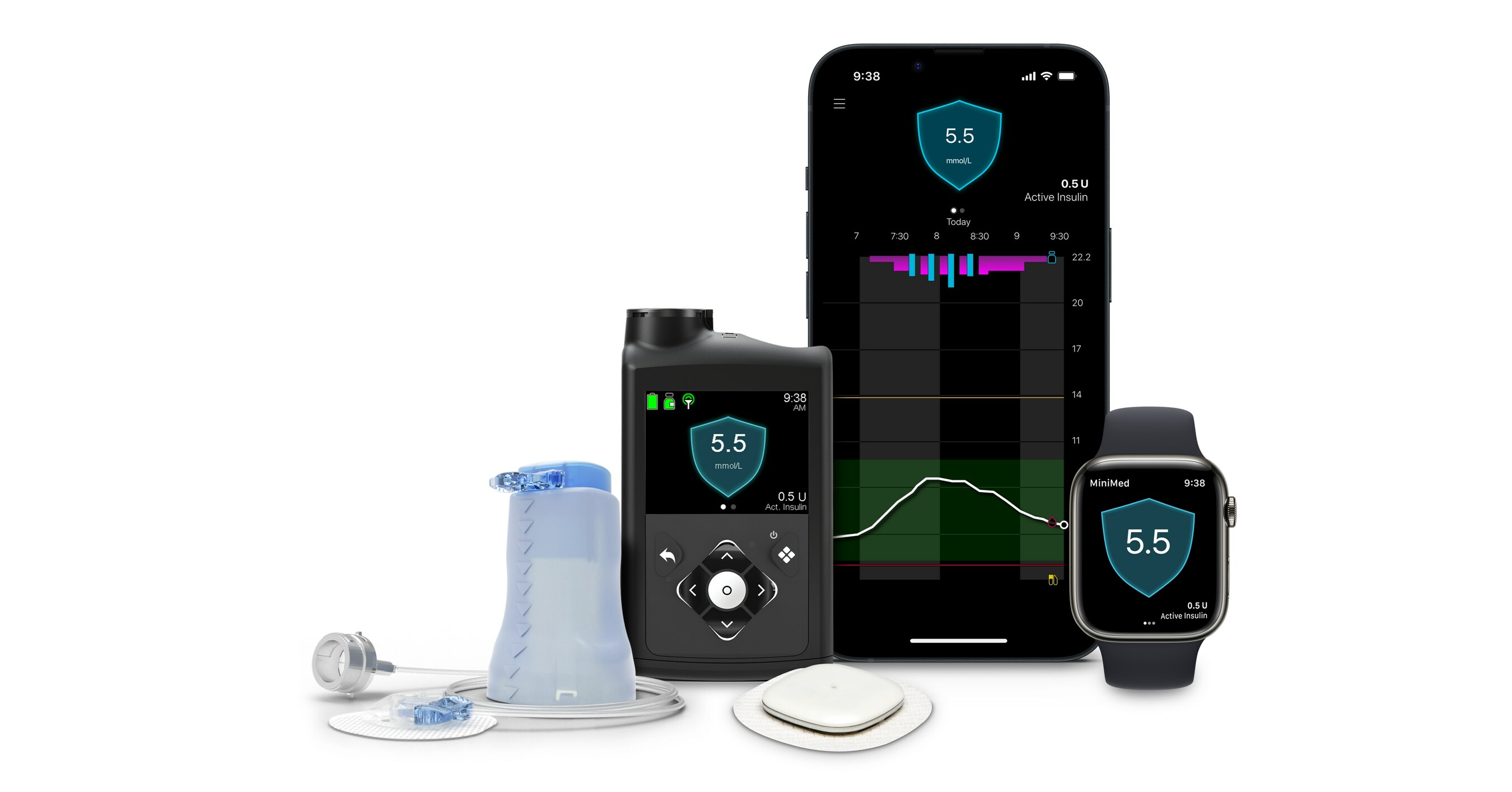
Medtronic Diabetes announces world's first approval for MiniMed™ 780G System with Simplera Sync™ disposable, all-in-one sensor
With CE Mark approval, the benefits of the MiniMed™ 780G system are now available with a new sensor that takes less than 10 seconds to insert1 DUBLIN, Jan. 8, 2024 /PRNewswire/ -- Medtronic plc...
Reading between the lines Medtronic know they made a lemon with the G4 and are not inclined to revise it, but instead put their money into re-designed their sensors from the ground up. The new sensor is half the size and is supposed to be easier to attach. It's now been approved in Europe but not yet in the US to the best of my knowledge. Likely to be available worldwide by summer, assuming FDA approval goes smoothly.
Again, I know nothing about these devices so I'm not sure this is useful information.
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
The new sensor would be the Simplera and the Instinct, same insertion method as G7, Libre.....so easy to work withDisclaimer - I know nothing about this technology so I'm not certain this is helpful.
After reading this thread and as I happen to know someone who works at Medtronic HQ I made contact asking if there were any plans to revise the Guardian 4. He does not work in the diabetes device division. I was provided with a little info and directed to this press release:

Medtronic Diabetes announces world's first approval for MiniMed™ 780G System with Simplera Sync™ disposable, all-in-one sensor
With CE Mark approval, the benefits of the MiniMed™ 780G system are now available with a new sensor that takes less than 10 seconds to insert1 DUBLIN, Jan. 8, 2024 /PRNewswire/ -- Medtronic plc...news.medtronic.com
Reading between the lines Medtronic know they made a lemon with the G4 and are not inclined to revise it, but instead put their money into re-designed their sensors from the ground up. The new sensor is half the size and is supposed to be easier to attach. It's now been approved in Europe but not yet in the US to the best of my knowledge. Likely to be available worldwide by summer, assuming FDA approval goes smoothly.
Again, I know nothing about these devices so I'm not sure this is useful information.
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Yeah, that's why I have given up on those GS which are really such a pain in the ass....I've been moved on to Medtronic 780 since November, and I've found the whole experience with the peripherals to be an absolute nightmare.
I think I've had two Guardian 4 sensors last the full 7 days. I've also had to replace the transmitter after a fortnight. The Guardian 4 is simply not fit for purpose. I can't believe it had any sort of user testing whatsoever. Having to mess around with the tapes to keep it in place drives me up the wall. I was using Freestyle Libre 3 before switching - they could stay happily attached for a fortnight.
I'm fed up of ringing Medtronic every week to report another failed sensor. One night I had to go through three before I got a functioning one. I asked my diabetes team to change my pump but they said I'm stuck with this one now until April 2025.
Totally a torturing mental crisis to go through.
Libre 3 (14days) is really a way to go as a sensor besides Dexcom G7.... despite of not being able talking to 780G pump
So unfortunate.
Medtronic should start consider to working with others and keep up with the competition among others within this field
Hopefully a new sensor, that is fit for purpose and meets end user requirements will lob sooner rather than later, as the 780G software is actually very, very good. My TIR since upgrading from the 770G (in manual, because the G3 sucked, with a Libre 2 sensor) is now better than 85%, whilst at the same time I have been able to reduce my daily insulin use by 20%. But IMO that doesn't excuse, or negate the hassle and aggravation of having to deal with a sensor that is so difficult to install and live with, that it seems to have been created in the stone age.
Fingers crossed for a satisfactory replacement soon.
Fingers crossed for a satisfactory replacement soon.
Just a footnote if I may.
I have just done a quick search and it seems that Medtronic will be replacing the G4 with a sensor called Simplera A.K.A. Instinct. As I understand it, at this time it (Simplera) will have a 7 day wear time. At this point I cannot find a whole lot about definite release times, but I am relieved that they have something that will replace the G4.
That being said, it would not hurt if Medtronic did a little PR and e-mailed, or sent a text, or 2 to every G4 user, to bring them up to speed with respect to the status of the new sensor and perhaps take some steps to help them persevere with the G4 and the Medtronic's pump, until its' replacement has been approved and is available.
I have just done a quick search and it seems that Medtronic will be replacing the G4 with a sensor called Simplera A.K.A. Instinct. As I understand it, at this time it (Simplera) will have a 7 day wear time. At this point I cannot find a whole lot about definite release times, but I am relieved that they have something that will replace the G4.
That being said, it would not hurt if Medtronic did a little PR and e-mailed, or sent a text, or 2 to every G4 user, to bring them up to speed with respect to the status of the new sensor and perhaps take some steps to help them persevere with the G4 and the Medtronic's pump, until its' replacement has been approved and is available.
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Exactly, Stone Age.. GS is almost 2 decades agoHopefully a new sensor, that is fit for purpose and meets end user requirements will lob sooner rather than later, as the 780G software is actually very, very good. My TIR since upgrading from the 770G (in manual, because the G3 sucked, with a Libre 2 sensor) is now better than 85%, whilst at the same time I have been able to reduce my daily insulin use by 20%. But IMO that doesn't excuse, or negate the hassle and aggravation of having to deal with a sensor that is so difficult to install and live with, that it seems to have been created in the stone age.
Fingers crossed for a satisfactory replacement soon.
you just ripped those words out of my mind.....
Just thought I'd vent. Another sensor fail. It's going to be a very long night for me, as it's alreaday 2150hrs on a warm Wednesday night and I am on hold waiting for the Medtronics people to do me the courtesy of picking up their phone and talking to me. If and when I change it out, it is another 2 hours min till it warms up and enters smart mode. This is such a regular occurrence, that I am incandescent with both impotence and rage. My 15 minute wait so far is not helping my mood.
Last edited:
One hour and fifteen minutes and I think I will quit while I am ahead.
THIS IS NO WAY TO SERVICE A PRODUCT THAT HAS LIFE AND DEATH IMPLICATIONS!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
THIS IS NO WAY TO SERVICE A PRODUCT THAT HAS LIFE AND DEATH IMPLICATIONS!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
MoonCloudy
Member
- Messages
- 17
- Type of diabetes
- Type 1
- Treatment type
- Pump
Its RIDICULOUS
Its really stressful and anxious, it will certainly not do you any good in terms of emotional "roller coaster" its something real bad for us as a consequence.
We should not ignore our emotional "breakdown" if it comes to this ongoing issue, it could make some "impact" on us, emotionally as well as physically.
Still hanging on something which is not doing its work properly or should work, needs to be considered seriously and try to look for other possibly options.
At least, this is what I will do and have done.
The reason was, consciously knowing I was not heading the right way with all these kind of stress and anxiety that I have been given to with this not "working well" GS.
Of course, there are consequences as well, like less time in range and having more high and lows and more fluctuations.
Thats a compromise you will need to commit to.
Feeling sick emotionally is not easy as well
Its really stressful and anxious, it will certainly not do you any good in terms of emotional "roller coaster" its something real bad for us as a consequence.
We should not ignore our emotional "breakdown" if it comes to this ongoing issue, it could make some "impact" on us, emotionally as well as physically.
Still hanging on something which is not doing its work properly or should work, needs to be considered seriously and try to look for other possibly options.
At least, this is what I will do and have done.
The reason was, consciously knowing I was not heading the right way with all these kind of stress and anxiety that I have been given to with this not "working well" GS.
Of course, there are consequences as well, like less time in range and having more high and lows and more fluctuations.
Thats a compromise you will need to commit to.
Feeling sick emotionally is not easy as well
2300hrs and I finally got someone to talk to. Not a lot of joy there, as the end result was that they will send me a replacement, as a courtesy, free. After warm up I finally went to bed about 0130hrs, tired and grumpy. Don't even know how this sensor made it past the product evaluation phase.
MoonCloudy, I have gone well beyond the stress phase with these G4 sensors. Today I went out and bought, at full retail, two Libre2 sensors. They are actually part of my problem, as I went from dumping the G3 and using the my pump in manual, with manual BG readings, to using the Libre2 sensor, still with the pump in manual. The Libre 2 is simple to install, easy to use, is small and robust and lasts 14 days, with pretty sound software that was easily installed on my phone.
I was then asked to do the software upgrade to the 780 on my pump and for that it was required I use the G4 sensor. As you will probably note from my previous posts, I am about at the end of my patience with this thing. IMO, I really do believe that Medtronic forgot to factor in the poor ******* who have to live with these sensors (G3 and G4) before they put them out to production.
Let's hope they get that new sensor approved lickity split.
Stay safe and sane mate.
P.S. I agree with what you are saying MoonCloudy. There are serious consequences with the sensors, as they are not toys, they are necessary medical devices and need to perform as advertised first time every time. I wouldn't like to see what these things do to my blood pressure haha.
I was then asked to do the software upgrade to the 780 on my pump and for that it was required I use the G4 sensor. As you will probably note from my previous posts, I am about at the end of my patience with this thing. IMO, I really do believe that Medtronic forgot to factor in the poor ******* who have to live with these sensors (G3 and G4) before they put them out to production.
Let's hope they get that new sensor approved lickity split.
Stay safe and sane mate.
P.S. I agree with what you are saying MoonCloudy. There are serious consequences with the sensors, as they are not toys, they are necessary medical devices and need to perform as advertised first time every time. I wouldn't like to see what these things do to my blood pressure haha.
