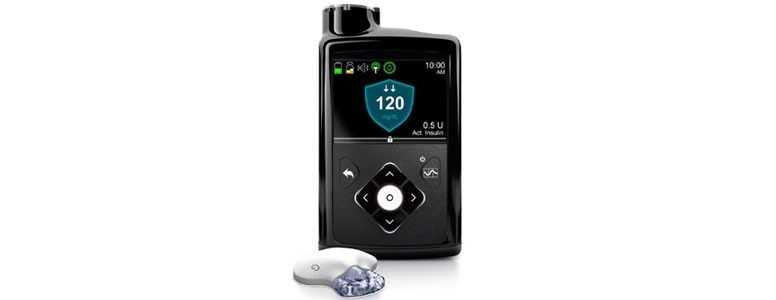
Medtronic’s MiniMed 670G has become the world’s first “hybrid closed-loop system” to secure the CE Mark, making the device available for sale in the European Union.
By receiving the CE Mark the MiniMed 670G meets the health, safety, and environmental protection standards for products sold within Europe.
It is now expected the device will be available in select European countries towards the end of 2018.
The MiniMed 670G, developed to make the lives of people with type 1 diabetes easier, was in 2016 approved for use in the US by the Food and Drug Administration (FDA) and launched in 2017.
The system combines an automated insulin pump with a continuous glucose monitor, and self-adjusts blood sugar levels and delivers basal insulin when needed.
The device uses a sensor called Guardian 3, which is inserted under the skin and thought to be 80% smaller than its predecessor, the Enlite. It also lasts an entire week, allowing for prolonged measurements of blood sugar levels.
Dr Pratik Choudhary, senior lecturer and consultant in diabetes at King’s College London, said: “We have seen that this innovation offers great promise for better glucose control and improved quality of life for those living with type 1 diabetes.
“The ability of the MiniMed 670G system to stabilise glucose levels automatically is an important advancement, and I look forward to introducing it to my patients in Europe.”
Alejandro Galindo, president of the Advanced Insulin Management division within the Diabetes Group at Medtronic, said: “The MiniMed 670G system advances our ability to automatically suspend and resume insulin delivery to the next level by automating self-adjusting basal insulin delivery every five minutes based on sensor glucose values – providing the most advanced algorithm available to deliver leading clinical outcomes.”
The system has been through a series of vigorous tests to ensure it is safe for people to use. Earlier this year the device was shown to help children better manage their type 1 diabetes without experiencing severe hypos or diabetic ketoacidosis.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.