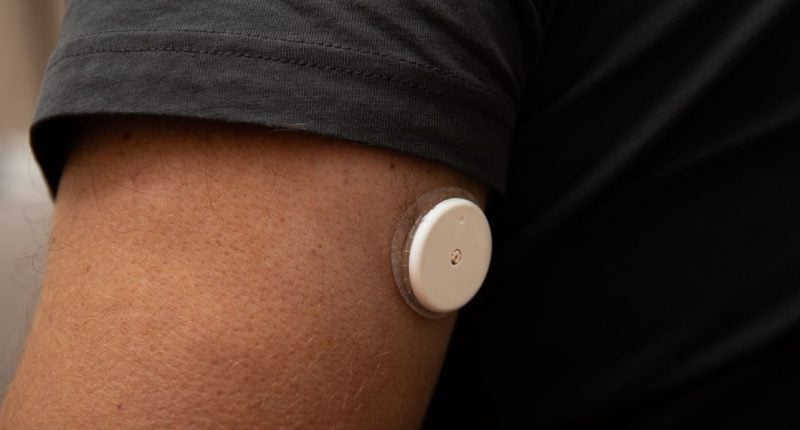
- A subset of FreeStyle Libre 3 and Libre 3 Plus sensors can give falsely low glucose readings
- Only specific Libre 3 and Libre 3 Plus sensors are affected.
Abbott, the manufacturer of FreeStyle Libre, has issued an urgent safety notice for some FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors after finding that certain sensors can give falsely low glucose readings.
Incorrect low readings can lead people to eat extra carbohydrate or delay or skip insulin, which can push glucose levels too high over time.
That has been linked to serious complications in some users.
Globally, Abbott has reported 736 serious adverse events and seven deaths that may be associated with this issue, although none of the deaths were in the United States.
The United States Food and Drug Administration has issued an early alert describing this as a potentially high risk problem.
A field safety notice and similar communications have been issued in the UK.
Which Libre products are affected
Only certain FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors are involved:
- FreeStyle Libre 3 sensor
- Model numbers: 72081 01, 72080 01
- Unique device identifiers (UDI DI): 00357599818005, 00357599819002)
- FreeStyle Libre 3 Plus sensor
- Model numbers: 78768 01, 78769 01
- Unique device identifiers (UDI DI): 00357599844011, 00357599843014)
Not affected
- FreeStyle Libre 14 day
- FreeStyle Libre 2
- FreeStyle Libre 2 Plus
- FreeStyle Libre Pro sensors
- FreeStyle Libre readers and mobile apps
- Other Abbott biowearables
How to check if your Libre 3 sensor is affected
You need the sensor serial number. You can find it in several places.
In the FreeStyle Libre 3 app
- Open the main menu
- Select About
- Look under Last 3 Sensors – the top entry is the sensor you are currently wearing or most recently used
In the Libre 3 reader
- Go to Settings
- Choose System Status, then System Info
- Look under Sensor SN and Status – number (1) is the current or most recent sensor
On the box and applicator
- The serial number is printed on the sensor carton
- It is also on the label on the bottom of the sensor applicator

What to do if your Libre 3 sensor is affected
1. Check your sensor on FreeStyleCheck
- Visit www.FreeStyleCheck.com
- Select Confirm sensor serial number
- Enter your sensor serial number
If your sensor is in an affected batch, the site will ask for your contact details so Abbott can send a free replacement.
This applies whether you get Libre on prescription or self fund.
2. Stop using an affected sensor
If a sensor you are wearing is confirmed as affected, remove it and dispose of it as you normally would
Do not continue to dose insulin based solely on that sensor’s readings
3. Use an alternative to guide treatment
Use the built in meter in the Libre 3 reader or use a separate blood glucose meter while you are waiting for a replacement
Always double check with a finger prick test if the sensor reading does not fit how you feel
4. Contact Abbott or your diabetes team if you are unsure
In the UK, Abbott’s customer service line for Libre is 0800 170 1177 (free from UK landlines and mobiles).
You should also contact your diabetes team urgently if:
- You feel unwell or have symptoms of high glucose such as excessive thirst, passing urine more often or unexplained tiredness
- You have any signs of diabetic ketoacidosis, such as nausea, vomiting, abdominal pain, deep or rapid breathing or breath that smells of pear drops
If you think you are in immediate danger, call the emergency services.
We will update this page if UK guidance changes.