New treatment options for diabetic macular oedema and for managing type 2 diabetes have been approved by the Scottish Medicines Consortium (SMC).
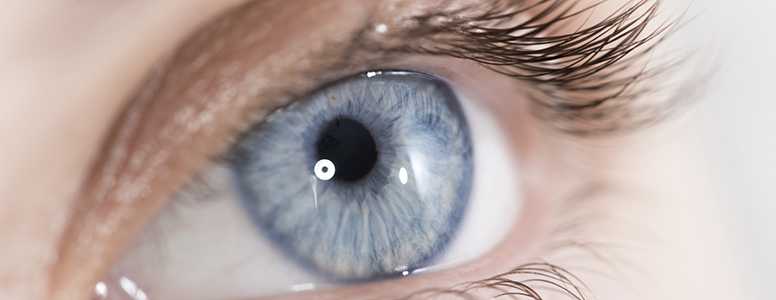
Diabetic macular oedema can lead to trouble reading and problems with our central vision. It is a condition that can result from diabetic neuropathy and affects approximately seven per cent of people with diabetes.
Dexamethasone 0.7mg intravitreal implant and Ozurdex, its applicator, will now be used by NHS Scotland in patients who have not responded to, or are unsuitable for other types of treatment.
According to SMC recommendations, Ozurdex will also apply to patients who have an artificial lens following >cataract
Ozurdex, developed by Allerga, is designed to reduce the burden on patients with diabetic macular oedema, with treatment lasting around five months, on average.
This should involve fewer clinic trips to patients compared to anti-VEGF therapies which can require monthly injections.
Two medicines were also approved by the SMC to treat type 2 diabetes: Victoza and Trajenta, which are given in combination with insulin.
Victoza is a once-daily medicine that can improve blood sugar levels in adults with type 2 diabetes, while Trajenta is also designed to improve glycemic control.
The SMC had previously accepted Victoza and Trajenta for use in combination with a variety of oral medicines for diabetes.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.